Steripharm labelling is designed to meet all applicable international standards and regulations. Where possible, Steripharm adopted the use of symbols to communicate requirements, product characteristics and provide guidance on handling and storage to the user. A compiled listing of symbols that may appear on the product labelling and the meaning of the symbol is provided in this document.

Indicates the authorized representative in the European Community.
Reference: SS-EN ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements (ISO 15223-1:2016) Ref no: 5.1.2

Indicates the date when the medical device was manufactured.
Reference: SS-EN ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements (ISO 15223-1:2016). Ref no: 5.1.3

Indicates the date after which the medical device is not to be used.
Reference: SS-EN ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements (ISO 15223-1:2016). Ref no: 5.1.4
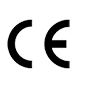
Indicates manufacturer declaration that the product complies with the essential requirements of the relevant European health, safety and environmental protection legislation.
European Medical Devices Directive 93/42/EEC of 14 June 1993 (as amended by Directive 2007/47/EC). As described in Article 17 of the Directive.

Indicates manufacturer declaration that the product complies with the essential requirements of the relevant European health, safety and environmental protection legislation.
European Medical Devices Directive 93/42/EEC of 14 June 1993 (as amended by Directive 2007/47/EC) As described in Article 17 of the Directive.

Indicates the manufacturer’s batch code so that the batch or lot can be identified.
SS-EN ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements (ISO 15223-1:2016). Ref no: 5.1.5

Indicates the manufacturer’s catalogue number so that the medical device can be identified.
SS-EN ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements (ISO 15223-1:2016. Ref no: 5.1.6

Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC
Reference: SS-EN ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements (ISO 15223-1:2016). Ref no: 5.1.1

Indicates a medical device that has been sterilized using ethylene oxide.
SS-EN ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements (ISO 15223-1:2016). Ref no: 5.2.3

Indicates a medical device that should not be used if the package has been damaged or opened.
SS-EN ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements (ISO 15223-1:2016). Ref no: 5.2.8.

Indicates a medical device that needs protection from light sources.
SS-EN ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements (ISO 15223-1:2016). Ref no: 5.3.2

Indicates a medical device that needs to be protected from moisture.
SS-EN ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements (ISO 15223-1:2016). Ref no: 5.3.4

Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure.
SS-EN ISO 15223-1:2016 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements (ISO 15223-1:2016). Ref. no: 5.4.2